Redox polymer electrodialysis system for PFAS removal. Credit: Nature Communications (2024). DOI: 10.1038/s41467-024-52630-w
Research at the University of Illinois at Urbana-Champaign aims to use electricity to capture, concentrate and destroy a diverse mixture of chemicals known as PFAS (including the increasingly popular very short-chain PFAS) from water in a single process. He was the first to explain chemical strategies.
This new development is poised to address the growing industrial problem of contamination with per- and polyfluoroalkyl materials, particularly in semiconductor manufacturing. The research results were published in the journal Nature Communications.
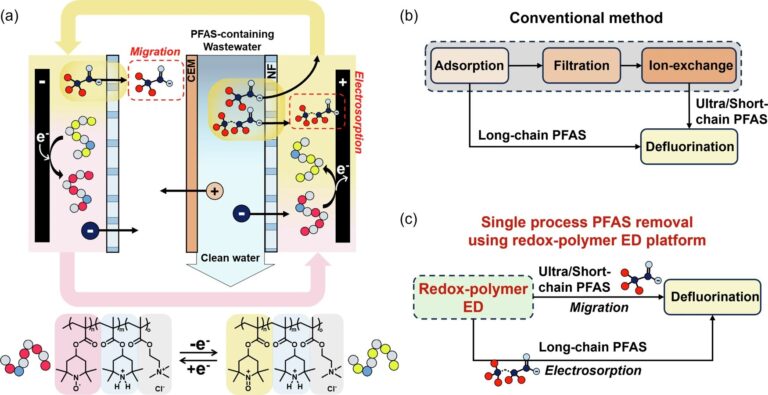
Previous research at the U. of I. showed that short- and long-chain PFAS can be removed from water using electrochemically driven adsorption, called electrosorption, but this method is small and has no effect on very short chain molecules. Different chemical properties.
The new research, led by Illinois chemical and biomolecular engineering professor Xiao Su, combines a desalting filtration technique called redox electrodialysis and electrosorption in a single device to capture the complete PFAS size spectrum. It addresses the problems involved.
“We decided to use redox electrodialysis because very short-chain PFAS behave much like salt ions in water,” Su said. “The challenge is to develop an efficient and effective electrodialysis system to capture ultra-short chain PFAS, work in conjunction with the electrosorption process of long-chain PFAS, and destroy them with electrochemical oxidation. , and to do it in a short amount of time on a single device.”
Su’s team has previously demonstrated highly efficient electrodialysis devices that remove a variety of non-PFAS contaminants. However, this process requires ion-exchange membranes, which are expensive and quickly fouled by PFAS molecules.
To overcome the membrane hurdle, Su’s team introduced an inexpensive nanofiltration membrane that can remove PFAS using electric fields without fouling. This technology builds on previous advances by their group to combine these nanofiltration membranes with redox polymers to enable energy-efficient desalination.
When it comes to PFAS removal, having the right materials for the job is important, but finding the most effective configuration is a challenge in itself.
“After experimenting with various equipment configurations, we finally settled on a system that desalinates PFAS-contaminated water to remove ultra-short chain molecules, while simultaneously using a carbon electrode to remove remaining short and long chain molecules. ,” Sue said. “This process also concentrates all PFAS, making it easier to destroy them after capture.”
Finally, the electrochemical oxidation process inherent in redox electrodialysis destroys the captured PFAS by converting them into fluoride ions. This is an important step in removing these residual contaminants from the environment.
Su said the team is excited at the prospect of not only taking this process out of the lab and into the field to address wastewater applications, but also scaling it up to be able to incorporate the system into industrial wastewater streams in the field. said.
“With interest from the U.S. government, wastewater treatment facilities, and the semiconductor industry, this effort is very timely,” Su said. “Semiconductor production is expected to increase in the coming years, and reducing PFAS for sustainable production will be a major challenge going forward.”
Further information: Nayang Kim et al. Integration of redox electrodialysis and electrosorption for the removal of ultra-short to long-chain PFAS, Nature Communications (2024). DOI: 10.1038/s41467-024-52630-w
Provided by University of Illinois at Urbana-Champaign
Citation: New PFAS removal process aims to eradicate contamination ahead of growth in semiconductor industry (November 7, 2024) https://phys.org/news/2024-11-pfas-aims Retrieved from -pollution-semiconductor-industry on November 7, 2024.html
This document is subject to copyright. No part may be reproduced without written permission, except in fair dealing for personal study or research purposes. Content is provided for informational purposes only.